Chemical bonding occurs when electrons from different atoms join together. Describe the general arrangement of subatomic particles in the atom.
The Structure Of The Atom Boundless Chemistry
Click card to see definition The nucleus consist of the protons and neutrons.
. How are the subatomic particles arranged in an atom according to modern structure of atom. What is the general arrangement of subatomic particles in the atom. The subatomic particles called protons and neutrons are located within the nucleus of an atom.
Because lithiums final electron goes into the 2 s subshell we write the electron configuration of a lithium atom as 1 s2 2 s1. Describe the general arrangement of subatomic particles in the atom. Check out a sample QA here.
Describe the arrangement of subatomic particles in an atom. The proton is located in the center or nucleus of an atom each atom has at least one proton. Electrons are subatomic particles.
Which means that the actual atoms had a sub-atomwhich is apart of the actual atom. Describe the general arrangement of subatomic particles in the atom. Most atoms have three different subatomic particles inside them.
Start your trial now. Subatomic particles are particles that are smaller than the actual particle. Electrons are arranged around the nucleus in energy levels or shells.
Was this answer helpful. When electrons are lost or gained from an atom ions are created. 1 c Calculate the average atomic mass of chlorine if its isotopes and percentage abundance are as follows.
The Bohr model shows the three basic subatomic particles in a simple manner. The nuclei of most atoms also contain neutrons. Describe the arrangement of subatomic particles in an atom.
There was a time when it was believed that atoms were the fundamental particles of matter and that they. Electrons are the subatomic particles that revolve around the nucleus of an atom. Protons neutrons and electrons.
Electrons are found moving around the nucleus. Most of the mass of the atom comes from the nucleus. Answer to b Describe the general arrangement of subatomic particles in the atom.
These electrons may be removed from or gained by an atom to form ions. Protons and neutrons are present in the nucleus. Foundations of College Chemistry 5th Edition Edit edition Solutions for Chapter 5 Problem 5E.
Electrons of different atoms come together to participate in chemical bonding. For our purposes we will concentrate only on three of them. The 2 s subshell holds a maximum of 2 electrons and the 2 p subshell holds a maximum of 6 electrons.
Answer to Describe the general arrangement of subatomic particles in. The subatomic particle known as. Subatomic particles are those which make up the atom.
But in the latter part of the 19th century and early part of the 20th scientists discovered that atoms are composed of certain subatomic particles and that no matter what the element the same. All matter in this universe is composed of atoms. It spins around an atoms nucleus.
Get the answers you need now. 3 Mass of Isotopes abundance 3696590 2447 3496885 7553 d 3 Determine the electron configuration notation for the following Ne. Most of an atom is empty space.
The protons and neutrons are packed together into the center of the atom which is called the nucleus and the electrons which are very much smaller whizz around the outside. A few points detailing the discovery and the properties of electrons are listed below. The next largest atom beryllium has 4 electrons so its electron configuration is 1 s2 2 s2.
B Describe the general arrangement of subatomic particles in the atom. Solution for Describe the general arrangement of subatomic particles in the atom. The structure of a.
82 755 Review Describe the general arrangement of subatomic particles in the atom. Click again to see term 17 Previous Next Flip Space. Ridhimamehra40 ridhimamehra40 3 weeks ago Science Primary School answered What is an atom.
Medium Solution Verified by Toppr Electrons revolve around the nucleus. Subatomic particles were discovered during the 1800s. Protons neutrons and electrons as seen in the helium atom below.
The atom is the smallest part of matter that represents a particular element. 0 0 Similar questions. Get solutions Get solutions Get solutions done loading Looking for the textbook.
Subatomic particles The nuclei of all atoms contain subatomic particles called protons. Want to see the full answer. First week only 499.
Describe the general arrangement of subatomic particles in the atom. A few points about the discovery of electrons and. Protons have a charge of positive one and a mass of approximately 1 atomic mass unit amu.
For quite a while the atom was thought to be the smallest part of matter that could exist. The word atom came from the Greek word Atomos which means non-divisibleThe atomic theory of matter was first proposed by John Dalton in 1808. Other particles exist as well such as alpha and beta particles which are discussed below.
A typical atom consists of three subatomic particles. Protons and Neutrons in the nucleus and electrons outside The chemical properties of atom ions and molecules are related to the. 2009-10-31 210524.

See The Electron Configuration Diagrams For Atoms Of The Elements Potassium Atom Atom Diagram Electron Configuration

Chemical Bonding Atomic Structure And Bonding Britannica
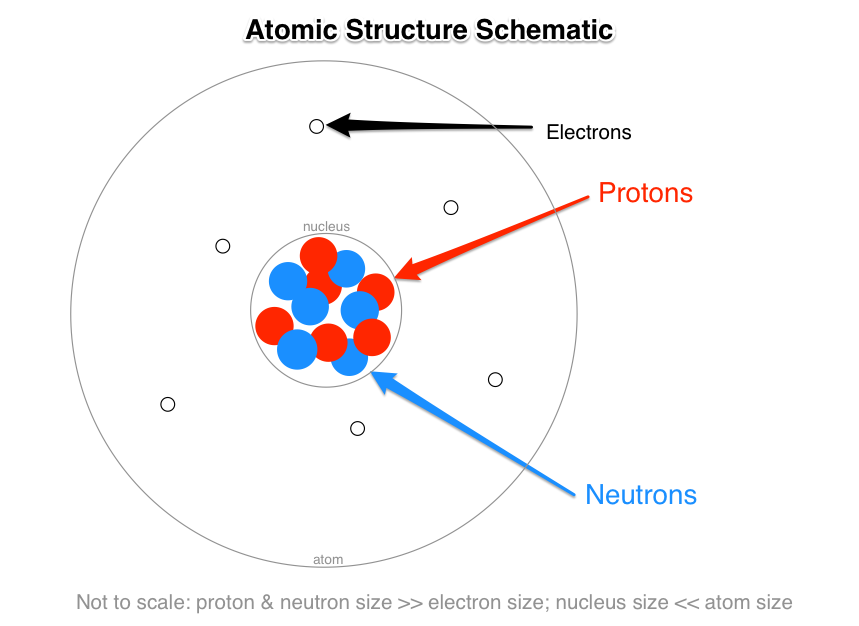
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic
0 Comments